Cell-cell interaction forces
SENSOCELL optical tweezers provide precise measurement of cell-cell interaction forces, facilitating a deeper insight into immune interactions, disease mechanisms, and cellular signalling.
Measuring cell-cell interaction forces using optical tweezers
Understanding cell-cell interaction forces is key for studying immune interactions, as these forces play a vital role in various physiological and pathological processes. Optical tweezers provide a powerful tool for trapping and manipulating cells, while direct force detectors based on light momentum analysis enable precise measurement of the forces exerted on the cells.
How to measure cell-cell adhesion forces:
First, we use the optical tweezers to trap and hold both cells in place using one optical trap for each cell. Next, the optical tweezers apply controlled trap motion to bring the cells into contact. SENSOCELL optical tweezers allow for precise control over the distance and orientation between the interacting cells and its integrated direct force detector allows measuring the interaction forces as the cells touch and interact. After a predefined interaction time, we move the cells apart and continue to measure the forces as they detach, thereby enabling the measurement of cell-cell adhesion force. Finally, the collected force and position data are used to generate force-distance and force-time curves, which provide detailed insights into the interaction forces at various stages of contact.
Selected publications:
- Glass DG, McAlinden N, Millington OR, Wright AJ. A minimally invasive optical trapping system to understand cellular interactions at onset of an immune response. PLoS One. (2017)
Immune cell-cell interaction forces of T-cells and cancer cells
In collaboration with Dr. Carlos Barcia Lab at the Universitat Autònoma of Barcelona (UAB), we demonstrate how Sensocell optical tweezers enable precise control of a trapped neuroblastoma cancer cell and a T-cell using two optical traps. In this example, we move the T-cell towards the cancer cell using the manual “click and drag” mode, establishing cell-cell contact. Next, after 10 seconds, the tweezers pull the T-cell back until the adhesion bond breaks. Throughout the process, the system records force and position data.
The accompanying figure and video illustrate the force dependence over time for the trap holding the T-cell. When the applied force is sufficient to break the bond between the two cells, the measured force drops to zero. In this case, the measured cell-cell adhesion force was 21 pN, consistent with the expected adhesion force of a single T-cell receptor.
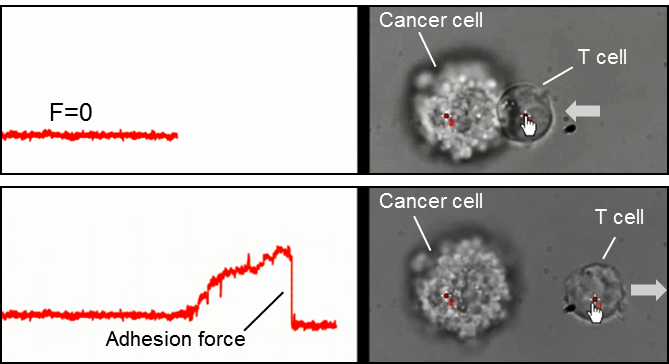
Fig.1 Cell-cell interaction between a cancer cell and a T-cell. The cell-cell adhesion force is given by the drop in the force signal (red line data) when the two cells are separated.
Video of the described cell-cell interaction assay
CAR-T cell and cancer cell interaction force
Chimeric Antigen Receptor T-cells (CAR-T cells) are genetically engineered T-cells designed to target and destroy cancer cells. SENSOCELL optical tweezers enable studying the interaction forces between CAR-T cells and cancer cells with high precision controlling parameters such as the interaction time and orientation. In this way, we can measure the adhesion force when the CAR-T cell binds to the cancer cell, its binding probability and detect multiple binding and unbinding events.
This information can help understand the biophysical mechanisms of how CAR-T cells recognize, bind to, and kill cancer cells. Insights gained from these measurements can help predict the success of CAR-T cell therapies by understanding the strength and stability of the interactions between different CAR-T cells and cancer cells. This is a valuable knowledge for developing new drugs that can enhance the effectiveness of CAR-T cell therapies by modulating their interaction forces.
In this example in collaboration with Dr. Juan Manel Otero from the Hospital Clínic of Barcelona, we used SENSOCELL optical tweezers to quantify cell-cell interactions between CAR-T cells and Lymphoma cancer cells. The cell-cell interaction time was set to 15 seconds using automated trap motion routines. The obtained interaction force traces often display multiple unbinding events. For example, here we observe two distinct unbinding events (see Fig. 1).
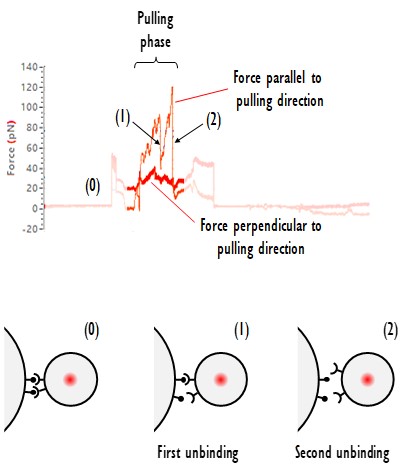
Fig.1 Cell-cell interaction force over time for an assay involving a CAR-T cell and a lymphoma cancer cell.
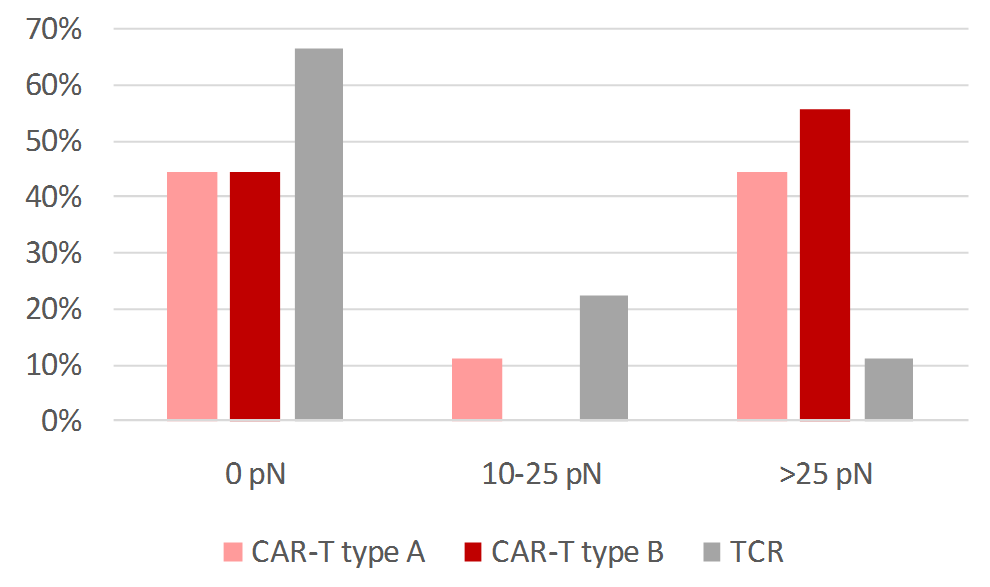
Fig.2 Statistics of cell-cell adhesion forces measured for lymphoma cancer cells interacting with T-cells and CAR-T cells expressing different cell membrane receptors. Each bar in the graph represents data from 9 different samples of each cell type.
Key Concepts
- T-cell: a type of white blood cell that plays a decisve role in the immune system. T-cells are involved in recognizing and responding to pathogens, infected cells, and cancer cells.
- CAR-T cell: a genetically engineered T-cell that has been modified to express a Chimeric Antigen Receptor (CAR) on its surface. This receptor allows CAR-T cells to specifically recognize and bind to cancer cells with particular antigens. CAR-T cell therapy is a form of immunotherapy used to treat certain types of cancers by harnessing these engineered cells to target and destroy cancerous cells more effectively.
- Cell-cell immune interactions: these refer to the interactions between immune cells (such as T-cells, B-cells, and antigen-presenting cells) and other cells, including pathogens, infected cells, or cancer cells. These interactions involve signaling molecules and surface receptors that enable immune cells to recognize, communicate with, and exert effects on target cells, playing a crucial role in immune responses and maintaining overall immune system function.
Advantages
- Cell force measurements: SENSOCELL’s direct force sensor allows measuring forces directly applied on cells, without any previous calibration.
- Precision: optical tweezers offer precise control over the position and movement of individual cells, allowing for detailed manipulation and measurement of cell-cell interactions.
- Sensitivity: SENSOCELL can measure very small forces in the picoNewton (pN) range, which is ideal for detecting the subtle forces involved in cell-cell interactions.
- Non-invasive: optical tweezers are minimally invasive, reducing potential damage to the cells and allowing for measurements in near-physiological conditions.
- Temporal resolution: the ability to measure forces in real-time provides dynamic insights into the interaction processes as they occur.
- Multifunctional: SENSOCELL optical tweezers can be combined with other techniques, such as fluorescence microscopy, to correlate mechanical measurements with biochemical signals.
Conclusions
Sensocell optical tweezers, with its direct force detectors based on light momentum analysis, provide a powerful and precise method for studying cell-cell interaction forces. Its distinctive technology allows measuring forces on cells and offers significant advantages in precision, sensitivity, and non-invasive measurement capabilities.
Would you like a DEMO?
Download SENSOCELL brochure
