Innovative Optical Tweezers TimSOM Microrheology Discovers Novel Disease Indicators in Aging Animals
TimSOM Microrheology: A New Technique for Optical Tweezers
The TimSOM Microrheology technique developed by IMPETUX and ICFO allows to measure viscoelasticity of biological materials in a simpler and more versatile way. The technique has reported, in a Nature Nanotechnology article, three novel results in the field of mechanobiology, for instance, that viscoelasticity of tissues inside living animals changes with age.
Understanding Viscoelasticity in Biological Materials
When mechanical stresses are applied, materials may deform elastically, like rubber, or flow like viscous liquids. The study of how materials respond is called rheology. Biological materials, however, are viscoelastic, meaning their properties depend on deformation speed and stress duration (think of silly putty).
In biology, viscoelastic changes are linked to severe diseases like cancer. Understanding the rheological properties of biological samples such as organelles, cells, and tissues is key to uncovering their physiological functions. This knowledge could also accelerate drug discovery, improve disease diagnosis, and provide insights into everyday materials like food, toothpaste, and lubricants.
Advancing Rheology with Optical Tweezers
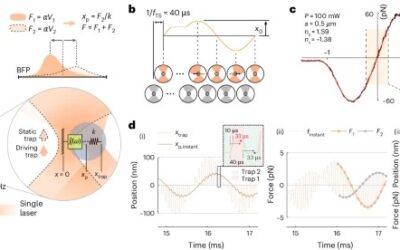
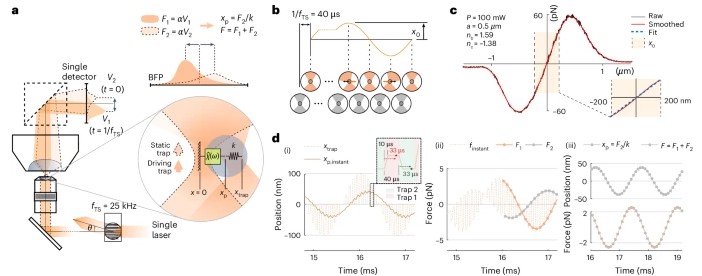
To address the challenges of studying biological materials, IMPETUX, ICFO, and collaborators have developed TimSOM (Time-Shared Optical Microrheology), a novel technique using optical tweezers. Published in Nature Nanotechnology, TimSOM simplifies and enhances rheological studies by requiring only a single laser, eliminating the complexity of previous dual-laser setups.
Key Advantages of TimSOM:
- Simplified Setup: A single-laser design reduces costs and complexity.
- User-Friendly: Includes a step-by-step protocol for User-friendly protocol for researchers in molecular, cellular, and developmental biology..
- Versatile Applications: Suitable for studying cells, tissues, and live organisms.
Groundbreaking Discoveries in Mechanobiology enabled by TimSOM:
- Protein Condensates: Using TimSOM, researchers studied protein condensates known to transition from liquid to solid states with age. They discovered that viscoelasticity is significantly higher inside mature condensates than at their interface, revealing insights into mechanisms linked to neurodegenerative diseases.
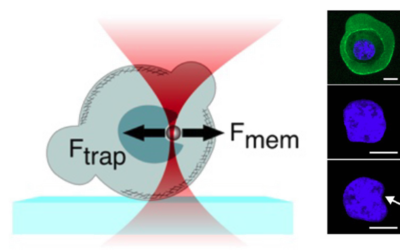
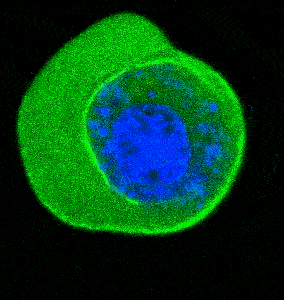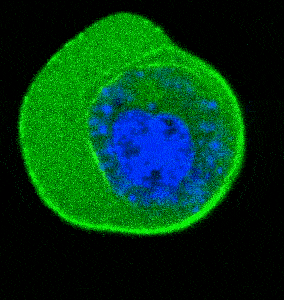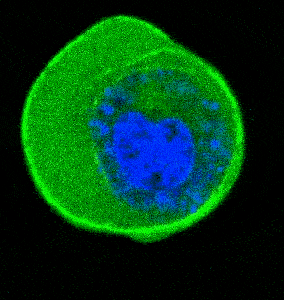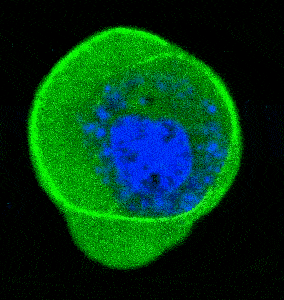
- Cellular Mechanics: Using cells from zebrafish embryos, TimSOM revealed that the nucleus-cytoplasm interface is stiffer than the cytoplasm, while the nucleoplasm is unexpectedly soft.
- Ageing and Viscoelasticity: In live C. elegans, TimSOM showed that tissue viscoelasticity changes with age. Mutations in the nuclear envelope that accelerate aging also affected viscoelasticity, offering new insights into aging processes.
Overcoming Challenges with TimSOM
TimSOM works by splitting a single laser into two optical tweezers: one applies force to the sample, while the other measures the resulting displacement. This setup reduces instrumentation complexity, measurement time, and costs.
However, time-sharing the laser introduced a slight delay between stress and strain measurements. To address this, the team developed a novel theoretical framework that retrieves the missing data from the raw measurements, ensuring precise results.
A Versatile Tool for Biological Research
According to first co-author Frederic Català-Castro, TimSOM’s single-laser system enables measurements across multiple locations in living cells, greatly enhancing spatiotemporal versatility. Co-inventor Dr. Paolo-Antonio Frigeri notes that this innovation reduces technical barriers, making optical tweezers-based microrheology more accessible than ever.
Reference:
Català-Castro et al., Measuring age-dependent viscoelastic properties of organelles, cells and organisms via Time-Shared Optical Tweezer Microrheology, Nature Nanotechnology (2025).
DOI: 10.1038/s41565-024-01830-y